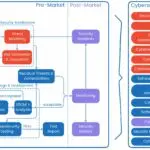
Medical Device Cybersecurity Resources
Download our comprehensive cybersecurity quick-start guide, or check out our blog posts on cybersecurity best practices for medical devices and FDA compliance.
Search Blog Topics
Recent Blog Posts
A Guide to Post-Market Cybersecurity Management
Your Medical Device is Cleared. Your Security Job Just Started. Summary Introduction: The Post-Market Reality Check Your medical device just received FDA clearance. The champagne bottles are empty, the team is celebrating, and you’re ready to move on to the…
Cybersecurity Guidance Update from FDA on June 27, 2025
FDA Updates Cybersecurity Guidance: Key Changes and Clarifications The FDA released an updated version of its cybersecurity guidance document for pre-market submissions on June 27, 2025, marking the first revision since the original September 2023 release. Below you’ll find redlined…
SPDF vs IEC 62304: Avoid Costly Documentation Mistakes
How FDA’s Secure Product Development Framework Enhances Software Lifecycle Processes While Requiring Separate Documentation Packages TL;DR Understanding SPDF vs IEC 62304 is crucial for medical device teams navigating today’s regulatory landscape. SPDF (Secure Product Development Framework) is “a set of…
Security Architecture as the Foundation of your Medical Device
Failure to build your medical device on a secure architecture costs millions and puts patients at risk Poor security architecture choices early in device design create problems that last for years. When companies try to add security features after they…
Medical Device Risk Assessment using CVSS
Explore the innovative application of the Common Vulnerability Scoring System (CVSS) in medical device risk assessment.
Secure Your Medical Device with Cybersecurity Controls
Discover essential strategies for safeguarding your digital assets with our comprehensive guide on implementing cybersecurity controls.
How to Conduct Medical Device Penetration Testing
Discover the essential steps and best practices for conducting effective penetration testing.
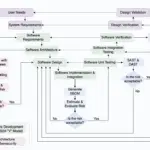
How to Implement Secure Software Development Lifecycle (SDLC) for Medical Devices
Discover the essential steps to integrate security into every phase of your software development lifecycle.
How to Perform security code review
Discover the essential steps and best practices for conducting a thorough security code review.
Master Data Flow Diagrams for Medical Devices: 4 Critical Components
Unlock the secrets of creating effective data flow diagrams tailored for medical devices.
How to Create an Architecture Security View for Your Medical Device
Learn how to develop a robust architecture security view for your medical device, aligning with FDA cybersecurity guidelines, AAMI TIR57, AAMI SW96, and industry best practices. Introduction As medical devices become increasingly interconnected, cybersecurity threats pose a significant risk to…
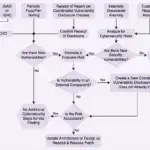
How to Establish Patch Management and Security Update Process
Learn how to create an effective patch management and security update process to protect your systems from vulnerabilities. This guide covers best practices, tools, and strategies to ensure your software and hardware are always up-to-date and secure.
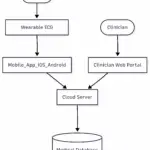
A Comprehensive Guide to Threat Modeling for Cloud-Connected Medical Devices
Learn how to perform threat modeling for cloud-connected medical devices leveraging FDA’s 2023 cybersecurity guidance and standards such as AAMI TIR57 and AAMI SW96. Introduction With the rise of connected healthcare, medical devices increasingly integrate embedded systems, mobile applications, and…
FDA Cybersecurity Requirements for Medical Devices with Software
FDA cybersecurity requirements apply to any medical device that has software. This surprises many MedTech executives. They assume that only cloud-connected medical devices have cybersecurity requirements. However, FDA’s guidance clearly states that’s not the case. With that said, as medical…
5 reasons FDA will refuse your 510(k) application due to cybersecurity deficiencies
Over the past decade, the FDA has steadily increased the degree of scrutiny applied to cybersecurity aspects of submissions. From the guidance issued on this topic in 2014, followed by extensive additions on the 2018 guidance and most recently the…