How FDA’s Secure Product Development Framework Enhances Software Lifecycle Processes While Requiring Separate Documentation Packages
TL;DR
Understanding SPDF vs IEC 62304 is crucial for medical device teams navigating today’s regulatory landscape. SPDF (Secure Product Development Framework) is “a set of processes that reduce the number and severity of vulnerabilities throughout the device lifecycle”—it enhances rather than replaces your existing IEC 62304 software development processes.
Key distinction: While SPDF integrates with IEC 62304 workflows, FDA requires separate documentation packages—software submissions need 9-10 attachments while cybersecurity submissions require 12 distinct attachments plus specialized testing documentation.
What you’ll learn: How to implement SPDF within existing IEC 62304 processes, coordinate dual documentation requirements, and leverage security activities that serve both software and cybersecurity submission needs without duplicating effort.
Table of Contents
Two Frameworks, One Goal
Software teams have spent years mastering IEC 62304’s disciplined approach to medical device software lifecycle processes. Your team follows established procedures for planning, requirements analysis, architectural design, and verification. Life was structured, predictable, and compliant.
Then FDA added new rules around cybersecurity.
Suddenly, your proven software development framework faces new challenges. The medical device cybersecurity framework requirements seem to demand entirely new processes, documentation, and expertise. Teams are asking: How do SPDF vs IEC 62304 requirements actually fit together?
The answer isn’t replacement—it’s intelligent enhancement. SPDF represents the security evolution of proven software lifecycle practices, designed to work alongside, not against, your existing IEC 62304 foundation.
What is SPDF and Why Software Teams Need It
SPDF Defined
The Secure Product Development Framework (SPDF) is FDA’s recommended approach for integrating cybersecurity throughout the device lifecycle. According to FDA’s 2023 cybersecurity guidance, SPDF is “a set of processes that reduce the number and severity of vulnerabilities in products throughout the device lifecycle.”
SPDF isn’t a competing standard—it’s a software lifecycle security overlay that complements existing frameworks. FDA explicitly recognizes that manufacturers may use frameworks like IEC 62304 that “satisfy the QS regulation and align with FDA’s recommendations for using an SPDF.”
The Documentation Reality: Separate But Coordinated
Here’s what many teams miss in the SPDF vs IEC 62304 discussion: while these frameworks work together operationally, they require distinct documentation packages for FDA submissions.
Software documentation requirements:
- Basic Documentation Level: 9 attachments
- Enhanced Documentation Level: 10 attachments
- Can leverage IEC 62304 Declaration of Conformity for lifecycle processes
Cybersecurity documentation requirements:
- 12 separate attachments regardless of software documentation level
- Plus cybersecurity labeling requirements
- Plus interoperability documentation
- Specialized testing reports (penetration testing, fuzz testing, etc.)
Understanding this distinction is crucial for resource planning and avoiding submission delays. Your secure software development lifecycle activities can inform both packages, but each requires specific deliverables.
The Dual Documentation Landscape
FDA’s Software Documentation Framework
FDA uses a risk-based approach to determine software documentation requirements. The Basic vs Enhanced Documentation Level decision depends on whether software failure could present “a probable risk of death or serious injury.”
Basic Documentation Level includes:
- Software Attachments
- Documentation Level Evaluation
- Software / Firmware Description
- Risk Management File
- Software Requirements Spec (SRS)
- System and Software Architecture Design (SAD) Chart
- Software Life Cycle Process Description
- Software Testing as Part of Verification & Validation
- Software Version / Revision Level History
- Unresolved Software Anomalies
Enhanced Documentation Level adds:
- Detailed Software Design Specifications (SDS)
- Enhancements to “Basic” documents:
- Complete configuration management documentation
- Unit and integration level test protocols
- More comprehensive verification evidence
Teams can satisfy some these requirements through IEC 62304 Declaration of Conformity, particularly for lifecycle processes, configuration management, and maintenance practices.
FDA’s Cybersecurity Documentation Requirements
FDA premarket cybersecurity submissions require a completely separate package focusing on security-specific considerations:
- Cybersecurity Management Plan – distinct from Software Development Plan
- Risk Management Report
- Threat Model – particularly for connected devices
- Cybersecurity Risk Assessment – using frameworks like CVSS
- Software Bill of Materials
- Software Level of Support
- Safety and Security Assessment
- Security Assessment of Unresolved Anomalies
- Cybersecurity Metrics
- Cybersecurity Controls
- Security Architecture Views – beyond basic software architecture
- Cybersecurity Testing
In addition, FDA requests information on Cybersecurity Labeling and Interoperability.
The Integration Opportunity
Smart teams recognize that while documentation packages are separate, the underlying SPDF activities can efficiently serve both requirements. Cybersecurity starts with security architecture planning that informs both software design and security documentation needs.
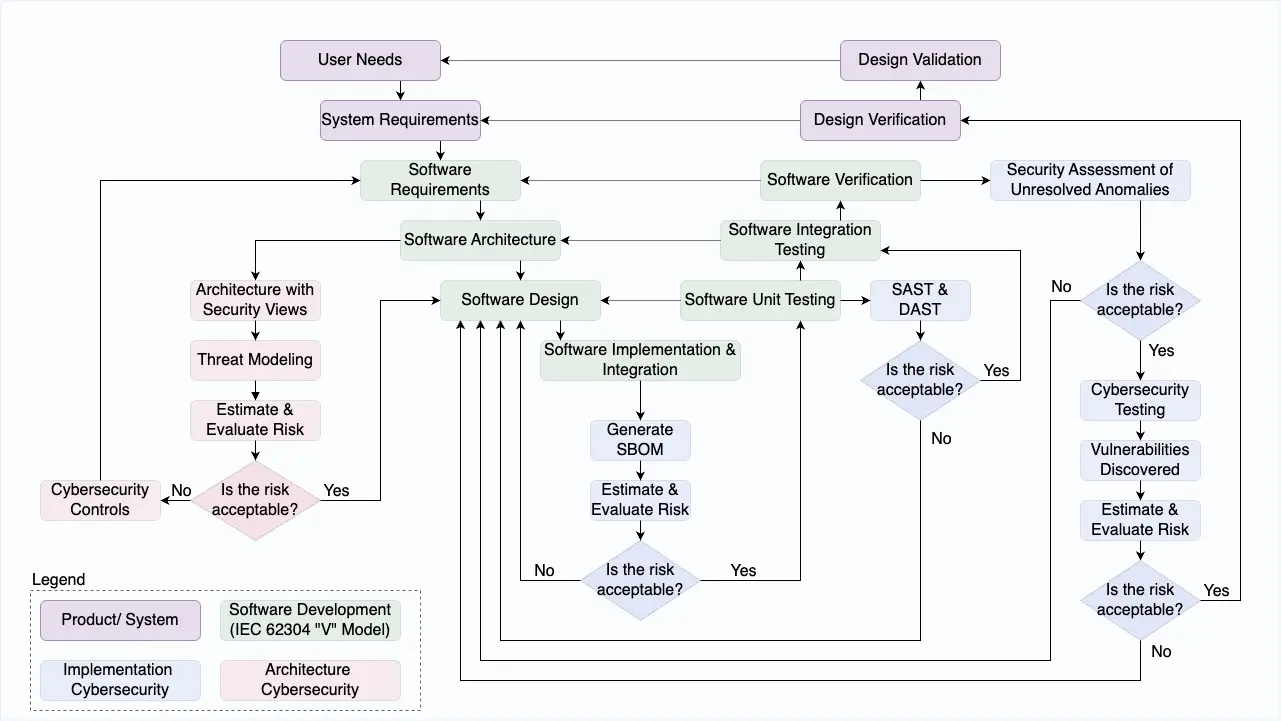
SPDF Integration Within IEC 62304 Processes
Software Development Planning Enhancement (IEC 62304 Section 5.1)
Enhanced Software Development Plan
Your existing IEC 62304 Software Development Plan can incorporate security considerations without losing its primary focus:
- Security risk management planning alongside traditional software risk management
- Security standards and tools identified within development methodology
- Security verification planning integrated with software verification activities
- Security configuration management within existing change control processes
The key insight in SPDF vs IEC 62304 integration: your enhanced Software Development Plan becomes more comprehensive while maintaining IEC 62304 compliance.
Separate Cybersecurity Management Plan Required
However, FDA cybersecurity requirements mandate a distinct Cybersecurity Management Plan addressing:
- Post-market vulnerability monitoring and identification processes
- Coordinated vulnerability disclosure procedures
- Customer communication protocols for security issues
- Security update deployment and patch management processes
- Integration with healthcare delivery organization security frameworks
This plan complements but doesn’t replace your Software Development Plan—think of it as the security lifecycle management companion to your software lifecycle management.
Software Requirements Analysis Security Integration (IEC 62304 Section 5.2)
Enhanced Software Requirements Specification
SPDF integration transforms requirements analysis by incorporating FDA’s five security objectives:
- Authenticity (including integrity)
- Authorization
- Availability
- Confidentiality
- Secure and timely updatability and patchability
These security requirements integrate naturally with functional requirements while maintaining clear traceability to both safety hazards and security threats.
Threat Modeling Integration
While IEC 62304 focuses on hazard analysis for safety, SPDF adds threat modeling for security. Teams can conduct both analyses using similar systematic approaches:
- Safety hazard analysis: “What could go wrong with the software?”
- Security threat analysis: “How could an attacker exploit the software?”
Both analyses inform requirements, but serve different risk management objectives.
Software Architectural Design Security Enhancement (IEC 62304 Section 5.3)
Security-Enhanced Architecture Documentation
Your software architecture documentation can satisfy both IEC 62304 and SPDF needs by including:
- Security domains and boundaries within the software architecture
- Security control placement and implementation strategy
- Data flow security considerations and data flow diagrams
- Interface security for internal and external connections
Separate Security Architecture Views
However, cybersecurity submissions require dedicated Security Architecture Views that go beyond software architecture:
- Global System View: Device within healthcare IT ecosystem
- Multi-Patient Harm View: Security controls preventing cross-patient impact
- Updatability and Patchability View: Secure update mechanisms
- Security Use Case View: Threat scenarios and mitigations
These views use your software architecture as foundation but focus specifically on security concerns.
Software Testing: Enhancement vs. Separation
Software Verification and Validation Enhancement
Your existing IEC 62304 testing processes can incorporate security verification:
- Unit testing: Include verification of security controls within existing unit test protocols
- Integration testing: Verify security interfaces and data protection during integration
- System testing: Validate security requirements alongside functional requirements
- Test traceability: Link security tests to security requirements and risk controls
This enhanced approach leverages existing testing infrastructure while ensuring security controls receive proper verification.
Separate Cybersecurity Testing Documentation
However, FDA requires specialized cybersecurity testing that goes beyond traditional software verification:
Vulnerability Testing Requirements:
- Fuzz testing for robustness validation
- Attack surface analysis to identify exposure points
- Vulnerability scanning using automated tools
- Static and dynamic code analysis including security code review
Penetration Testing Requirements:
- Independent testing: Documentation of tester independence from development team
- Comprehensive scope: Network, application, and physical security testing
- Detailed reporting: Methods, findings, and manufacturer assessment of results
- Remediation plans: How identified vulnerabilities will be addressed
This specialized testing complements but doesn’t replace software verification and validation—it provides security-specific validation that traditional testing cannot achieve.
Risk-Based Documentation Alignment
IEC 62304 Safety Classes Meet FDA Documentation Levels
Understanding how medical device cybersecurity framework requirements align with existing safety classifications helps teams plan efficiently:
Class A Software (No injury or damage possible):
- Usually qualifies for Basic Documentation Level
- Minimal security risk typically doesn’t elevate requirements
- SPDF integration focuses on foundational security practices
Class B Software (Non-life-threatening injury possible):
- May require Enhanced Documentation if cybersecurity risks are significant
- Security controls implementation becomes more rigorous
- Cybersecurity risk assessment can influence documentation level decision
Class C Software (Death or serious injury possible):
- Almost always requires Enhanced Documentation Level
- Comprehensive cybersecurity documentation expected
- Full SPDF implementation typically necessary
Security Risk Influence: In the SPDF vs IEC 62304 comparison, remember that cybersecurity threats can elevate documentation requirements beyond traditional safety classification. A Class A device with significant connectivity and data handling may require Enhanced Documentation due to cybersecurity risk.
Documentation Package Resource Planning
Basic Software + Full Cybersecurity Documentation:
- 9 software attachments + 12 cybersecurity attachments
- Streamlined software package but comprehensive security documentation
- Common for devices with lower software risk but significant connectivity
Enhanced Software + Full Cybersecurity Documentation:
- 10 software attachments + 12 cybersecurity attachments
- Maximum documentation burden requiring significant resources
- Typical for Class C software in connected devices
Teams should budget accordingly—cybersecurity documentation represents substantial additional effort regardless of software documentation level.
Lifecycle Management: Coordinated But Distinct
Software Maintenance Enhancement (IEC 62304 Section 6)
SPDF enhances existing software maintenance processes:
Problem Resolution Integration:
- Security vulnerability handling within existing software problem resolution
- Security impact assessment for software changes
- Coordinated approach to safety and security anomalies
Configuration Management Enhancement:
- Security patches managed through existing software configuration control
- Version control includes security-relevant changes
- Change impact assessment includes security implications
Change Control Integration:
- Security considerations integrated into existing change control processes
- FDA cybersecurity requirements addressed within established workflows
- Unified approach to software change management
Separate Post-Market Cybersecurity Requirements
However, cybersecurity lifecycle management requires distinct processes:
Cybersecurity Management Plan Execution:
- Vulnerability monitoring: Systematic surveillance of security threat landscape
- Threat intelligence: Integration with industry security information sources
- Security signal processing: Evaluation and triage of potential security issues
Coordinated Vulnerability Disclosure:
- Stakeholder notification: Communication with healthcare delivery organizations
- Industry coordination: Information sharing with other manufacturers and security organizations
- Regulatory reporting: Notification to FDA and international regulators as required
Customer Security Communication:
- Security advisories: Timely notification of security issues
- Patch deployment: Secure update delivery and installation
- Configuration guidance: Ongoing security configuration recommendations
Beyond Software: Cybersecurity-Specific Requirements
Cybersecurity Labeling Requirements
Traditional IEC 62304 processes don’t address cybersecurity labeling, but FDA requires comprehensive security information for users:
Instructions for Use Enhancement:
- Security configuration guidance: How to securely set up and operate the device
- Network requirements: Security prerequisites for device connectivity
- User security responsibilities: What healthcare organizations must do to maintain security
Vulnerability Contact Information:
- Clear reporting pathways: How to report suspected security vulnerabilities
- Contact mechanisms: Email, phone, or web form for security issues
- Response expectations: Timeline and process for security issue handling
End-of-Life Security Information:
- End-of-support notifications: When security updates will no longer be available
- Risk transfer process: How cybersecurity risks transfer to customers after support ends
- Decommissioning guidance: Secure disposal and data sanitization procedures
Interoperability Documentation
Connected medical devices require security analysis beyond individual software functionality:
System Integration Security:
- Healthcare IT ecosystem analysis: How device security integrates with hospital networks
- Data flow security: Protection of data in transit and at rest
- Third-party integration: Security implications of connected systems and services
Network Security Requirements:
- Connectivity prerequisites: Network security controls required for safe operation
- Interface security: Protection of communication interfaces and APIs
- Authentication and authorization: How device integrates with healthcare identity management
This interoperability analysis extends beyond traditional software architecture documentation and requires specialized cybersecurity expertise.
Implementation Strategy: Coordinated Development
Process Integration Planning
Unified Security Activities: The smartest approach to SPDF vs IEC 62304 implementation recognizes that single security activities can inform multiple documentation requirements:
- Security risk assessment: Serves both software risk management and cybersecurity risk assessment needs
- Security architecture design: Informs software architecture documentation and security architecture views
- Security testing: Provides evidence for both software verification and cybersecurity testing requirements
Resource Allocation Strategy:
- Cross-functional teams: Software and cybersecurity experts working together
- Shared deliverables: Security activities that serve multiple documentation needs
- Coordinated timelines: Parallel development of software and cybersecurity documentation
Tool Integration:
- Requirements management: Tools that handle both functional and security requirements
- Test management: Systems that coordinate software and security testing
- Document management: Platforms that maintain traceability across both documentation packages
Practical Implementation Steps
Phase 1: Assessment and Planning
- Current state analysis: Evaluate existing IEC 62304 implementation maturity
- Gap assessment: Identify where SPDF integration is needed
- Resource planning: Budget for dual documentation requirements
- Team preparation: Train existing software teams on security concepts
Phase 2: SPDF Integration
- Enhanced planning: Update Software Development Plans with security considerations
- Requirements integration: Incorporate security requirements into existing requirements management
- Architecture enhancement: Add security considerations to software architecture design
- Process integration: Embed security activities within existing software development workflows
Phase 3: Documentation Preparation
- Software documentation: Prepare enhanced IEC 62304-compliant documentation
- Cybersecurity documentation: Develop separate cybersecurity submission package
- Cross-reference management: Ensure traceability between software and security documentation
- Review and validation: Verify both packages meet FDA requirements
Phase 4: Continuous Improvement
- Process refinement: Optimize integration based on experience
- Tool enhancement: Improve systems for managing dual documentation requirements
- Team development: Build expertise in both software and cybersecurity domains
- Stakeholder feedback: Incorporate lessons learned from regulatory interactions
Cost-Benefit Analysis: Investment vs. Value
Understanding the Investment
Increased Documentation Burden: Teams must plan for significantly expanded documentation requirements:
- Dual submission packages: Software documentation (9-10 attachments) plus cybersecurity documentation (12 attachments)
- Specialized expertise: Security testing, threat modeling, and vulnerability assessment capabilities
- Ongoing maintenance: Post-market cybersecurity management and vulnerability response
- Training investment: Building team capabilities in both domains
Resource Implications: The SPDF vs IEC 62304 discussion isn’t just about technical integration—it’s about resource allocation. Cybersecurity adds substantial documentation and ongoing management requirements that teams must budget for appropriately.
Value Proposition
Regulatory Efficiency: Coordinated SPDF implementation provides significant benefits:
- Reduced duplication: Single security activities serving multiple documentation needs
- Faster submissions: Well-integrated processes reduce preparation time
- Lower rejection risk: Comprehensive approach reduces common FDA rejection reasons
- Market access: Meeting both software and cybersecurity requirements enables device approval
Operational Benefits:
- Risk mitigation: Comprehensive safety and security coverage protects patients and business
- Competitive advantage: Strong cybersecurity posture differentiates products in the market
- Future readiness: Preparation for evolving regulatory landscape and emerging threats
- Customer confidence: Healthcare organizations increasingly require strong cybersecurity
Long-term Value: While the initial investment is substantial, the medical device cybersecurity framework requirements are only going to become more stringent. Early investment in coordinated SPDF and IEC 62304 processes positions teams for long-term success.
Integration with Clear Boundaries
The SPDF vs IEC 62304 question isn’t really about choosing between frameworks—it’s about understanding how they work together while maintaining distinct requirements. SPDF enhances your existing software lifecycle processes while requiring separate cybersecurity documentation that goes far beyond traditional software development.
Key insights for implementation:
Process Integration: SPDF security activities should be embedded within existing IEC 62304 workflows wherever possible. Your Software Development Plan, requirements analysis, architectural design, and testing processes can all be enhanced with security considerations without losing their primary focus.
Documentation Separation: While processes integrate, documentation packages remain distinct. Plan for 9-10 software attachments plus 12 cybersecurity attachments, each requiring specific content and expertise.
Resource Planning: The dual documentation requirement represents significant additional effort. Budget accordingly and build cross-functional teams that can efficiently serve both software and cybersecurity submission needs.
Continuous Evolution: Both software lifecycle security requirements and cybersecurity threats continue evolving. Build processes that can adapt to changing regulatory expectations and emerging security challenges.
Future Readiness: Teams that successfully integrate SPDF with IEC 62304 now will be best positioned for future regulatory changes and market demands. The investment in coordinated processes pays dividends as cybersecurity requirements become more stringent.
Start with SPDF integration planning that serves both your software and cybersecurity documentation needs. Focus on security activities that efficiently support multiple submission requirements. Build the capability to manage dual documentation packages while leveraging the strong foundation your IEC 62304 processes already provide.
The future belongs to teams that can seamlessly blend software safety and security throughout the device lifecycle—without losing the discipline and structure that made their software development successful in the first place.
Need help with your cybersecurity or IEC 62304 process and documentation? Email us at [email protected].