Medical Device Cybersecurity Resources
Download our comprehensive cybersecurity quick-start guide, or check out our blog posts on cybersecurity best practices for medical devices and FDA compliance.
Search Blog Topics
Recent Blog Posts
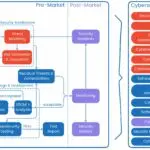
How to Create an Architecture Security View for Your Medical Device
Learn how to develop a robust architecture security view for your medical device, aligning with FDA cybersecurity guidelines, AAMI TIR57, AAMI SW96, and industry best practices. Introduction As medical devices become increasingly interconnected, cybersecurity threats pose a significant risk to…
How to Establish Patch Management and Security Update Process
Learn how to create an effective patch management and security update process to protect your systems from vulnerabilities. This guide covers best practices, tools, and strategies to ensure your software and hardware are always up-to-date and secure.
A Comprehensive Guide to Threat Modeling for Cloud-Connected Medical Devices
Learn how to perform threat modeling for cloud-connected medical devices leveraging FDA’s 2023 cybersecurity guidance and standards such as AAMI TIR57 and AAMI SW96. Introduction With the rise of connected healthcare, medical devices increasingly integrate embedded systems, mobile applications, and…
FDA Cybersecurity Requirements for Medical Devices with Software
FDA cybersecurity requirements apply to any medical device that has software. This surprises many MedTech executives. They assume that only cloud-connected medical devices have cybersecurity requirements. However, FDA’s guidance clearly states that’s not the case. With that said, as medical…
5 reasons FDA will refuse your 510(k) application due to cybersecurity deficiencies
Over the past decade, the FDA has steadily increased the degree of scrutiny applied to cybersecurity aspects of submissions. From the guidance issued on this topic in 2014, followed by extensive additions on the 2018 guidance and most recently the…
New Cybersecurity Regulations Make Remote Software Updates Practically Mandatory
A startup recently shared with us that they were considering incorporating remote software updates into their medical device. They knew this feature would add a lot of value, but were leaning against it for fear it would create barriers to…
Ensuring Secure Software Updates for Medical Devices
Secure software updates are essential for protecting medical devices. As technology continues to advance, the importance of ensuring security through software updates has become paramount. While remote software updates have traditionally been seen as a convenient feature, the FDA now…
What Information FDA is Expecting for Cyber Devices?
In an era where medical devices are increasingly interconnected and software-dependent, cybersecurity has become a critical component of healthcare. The FDA recognizes the importance of cybersecurity to protect public health and ensure the safety and effectiveness of medical devices. To…